Quantum mechanics is a fascinating field that has revolutionized our understanding of the world around us. One of the key concepts in this field is quantization, which refers to the idea that certain physical quantities can only take on certain discrete values. This concept underlies many of the strange and counterintuitive phenomena that we observe in the quantum world, from the behavior of electrons in atoms to the properties of light.
At its core, quantization is a reflection of the fundamental nature of matter and energy. In the quantum world, particles and waves are intimately intertwined, and the behavior of one can only be understood in relation to the other. This leads to a strange and often bewildering set of rules that govern the behavior of particles on the smallest scales, but also allows for the development of powerful technologies such as quantum computers and ultra-precise sensors. In this article, we will explore the concept of quantization in more detail, examining its origins, implications, and applications in the world of quantum mechanics.
What is Quantization in Quantum Mechanics?
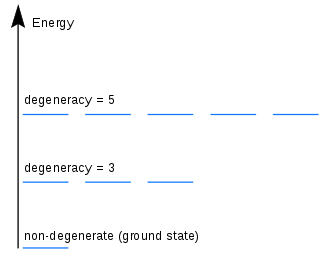
Quantization, in quantum mechanics, is the process of transitioning a classical mechanical system to a quantum mechanical system. This transition is often referred to as a “quantum leap.” In quantization, the energy of a particle or system is changed in discrete steps, or quanta, rather than in a continuous fashion. This process is also referred to as “energy quantization.”
Quantization is an important concept in physics and chemistry, as it helps to explain phenomena that occur in the atomic and subatomic world. It is also used to explain the behavior of light and other electromagnetic radiation. In addition, it is used to describe the behavior of particles in the quantum realm.
Quantized Energy
Quantized energy is the energy associated with a quantum state of a particle or system. It is the energy that is associated with a particular quantum state and it is quantized in discrete steps. This means that the energy of a particle or system can only change in discrete steps, rather than in a continuous fashion. This process is also known as energy quantization.
The quantization of energy is an important concept in quantum mechanics and is used to explain a wide range of phenomena including the behavior of light and other electromagnetic radiation. It is also used to explain the behavior of particles in the quantum realm.
Quantum Leap
A quantum leap is the transition of a classical mechanical system to a quantum mechanical system. This transition is often referred to as a “quantum leap” because it occurs in discrete steps, or quanta, rather than in a continuous fashion. This process is also referred to as “energy quantization.”
Quantum leaps are an important concept in quantum mechanics as they help to explain phenomena that occur in the atomic and subatomic world. They are also used to explain the behavior of light and other electromagnetic radiation. In addition, they are used to describe the behavior of particles in the quantum realm.
Quantum Entanglement
Quantum entanglement is a phenomenon that occurs when two particles are linked together, even if they are separated by a large distance. This phenomenon was first observed in the laboratory in 1935 and has since been demonstrated in a variety of experiments. It is an important concept in quantum mechanics and has implications for a wide range of fields, including quantum computing, cryptography, and communication.
In quantum entanglement, the state of one particle is dependent on the state of the other particle, regardless of their physical distance from one another. This phenomenon is a consequence of quantum mechanics and is an example of how quantum systems can exhibit behavior that is not possible in classical systems.
Quantum Mechanics
Quantum mechanics is a branch of physics that deals with the behavior of matter and energy at the atomic and subatomic level. It is a fundamentally new way of looking at the universe and is based on the idea that all matter and energy is quantized or divided into discrete particles. Quantum mechanics is an important field of physics and has implications for a wide range of fields, including chemistry, materials science, and condensed matter physics.
In quantum mechanics, the behavior of particles is described by the laws of quantum mechanics. These laws describe the behavior of particles in terms of probabilities rather than certainties. This means that particles do not necessarily follow a predictable path, but instead take a probabilistic route. This probabilistic behavior is one of the most remarkable aspects of quantum mechanics and has implications for a wide range of fields, from computing to cryptography.
Frequently Asked Questions (FAQs) about Quantization in Quantum Mechanics
Quantization in quantum mechanics is the process of expressing certain physical properties of a system in discrete units. It is the basis of the study of atomic and subatomic particles, and is essential for understanding the behavior of matter on the atomic scale.
What is Quantization in Quantum Mechanics?
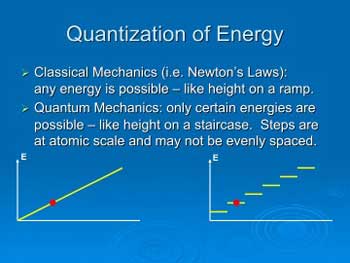
Quantization in quantum mechanics is the process of expressing certain physical properties of a system in discrete units. This means that instead of a continuous range of values, the values can only take on a certain number of distinct values. This is known as the “quantization” of a property. For example, a particle can only have certain energy levels, and can only take on certain values for its momentum. This is in contrast to classical mechanics, where properties can take on any value within a continuous range.
Quantization is essential for understanding the behavior of matter on the atomic scale, as it explains why certain properties of particles cannot be measured exactly, but only within certain limits. This leads to the concept of uncertainty, which is a fundamental feature of quantum mechanics.
What are the Benefits of Quantization in Quantum Mechanics?
Quantization in quantum mechanics allows us to understand the behavior of matter on the atomic scale. By quantizing certain physical properties of a system, we can gain insights into the behavior of particles and their interactions with each other. This helps us to understand how atoms and molecules interact, and how they form the basis of matter.
Quantization also allows us to make predictions about the behavior of matter on the atomic scale. By quantizing certain properties, we can use the rules of quantum mechanics to calculate the probability of certain outcomes. This means that we can make predictions about how particles will behave in a given situation. This is important for understanding the behavior of matter on the atomic scale, as it provides us with a useful tool for studying the behavior of matter.
How Does Quantization Work?
Quantization in quantum mechanics works by dividing a property into discrete units. This means that instead of a continuous range of values, the values can only take on a certain number of distinct values. For example, an electron can only have certain energy levels, and can only take on certain values for its momentum. The process of quantization assigns a certain number of “quanta” to a particle, which determines its properties.
Quantization is essential for understanding the behavior of matter on the atomic scale, as it explains why certain properties of particles cannot be measured exactly, but only within certain limits. This leads to the concept of uncertainty, which is a fundamental feature of quantum mechanics.
What are the Limitations of Quantization?
Quantization in quantum mechanics is a powerful tool for understanding the behavior of matter on the atomic scale, but it has certain limitations. Quantization is only able to describe the behavior of particles on the atomic scale, and is not able to explain the behavior of particles on larger scales. Additionally, the process of quantization is limited by the uncertainty principle, which states that certain properties of particles cannot be measured exactly.
Quantization also does not provide us with a complete picture of the behavior of matter on the atomic scale. It is only able to describe certain properties of particles, and does not provide us with a full understanding of their behavior. This means that we must rely on other methods, such as experiments and observations, to gain a complete understanding of the behavior of matter.
What is the Difference Between Classical Mechanics and Quantum Mechanics?
The main difference between classical mechanics and quantum mechanics is quantization. In classical mechanics, properties can take on any value within a continuous range. In quantum mechanics, certain properties of particles are quantized, meaning they can only take on a certain number of distinct values. This leads to the concept of uncertainty, which is a fundamental feature of quantum mechanics.
Another difference between classical mechanics and quantum mechanics is the scale at which they can be applied. Classical mechanics is used to describe the behavior of particles on larger scales, while quantum mechanics is used to describe the behavior of particles on the atomic scale. This means that quantum mechanics is essential for understanding the behavior of matter on the atomic scale.

Quantum Field Theory 4a – Second Quantization I
In conclusion, quantization in quantum mechanics is a fundamental concept that has revolutionized our understanding of the world around us. It explains how energy, matter, and other physical properties occur in discrete packets or quanta, rather than a continuous flow. This concept has transformed the way we perceive the behavior of subatomic particles and has led to the development of new technologies and scientific discoveries.
Overall, the concept of quantization has played a crucial role in shaping our understanding of the universe at its most fundamental level. As scientists continue to explore the mysteries of quantum mechanics, the concept of quantization will undoubtedly remain a cornerstone of this field. Whether through its applications in technology, or its role in advancing our understanding of the cosmos, the significance of quantization in quantum mechanics cannot be overstated.