Quantum mechanics is a fascinating and complex field that has revolutionized the way we understand the fundamental workings of our universe. However, with its many intricate concepts and technical jargon, it can be difficult to grasp some of the more abstract ideas associated with quantum mechanics. One such concept is degeneracy, which refers to the phenomenon where two or more quantum states share the same energy level.
In this article, we will delve into the world of quantum mechanics to explore what degeneracy is, how it arises, and why it is important. We will also examine some real-world examples of degeneracy and its practical applications in fields such as chemistry, physics, and engineering. So, whether you are a seasoned quantum mechanics expert or simply curious about the mysteries of the universe, read on to discover the fascinating world of degeneracy in quantum mechanics.
Degeneracy in quantum mechanics is a situation where multiple different states of a system have an identical energy. This occurs when the energy of a system is independent of certain quantum numbers due to symmetry. Degeneracy can be used to explain why there are many different elements in the periodic table with similar properties, and why electrons in an atom can occupy the same energy level.
Degeneracy in quantum mechanics is a situation where multiple different states of a system have an identical energy. This occurs when the energy of a system is independent of certain quantum numbers due to symmetry. Degeneracy can be used to explain why there are many different elements in the periodic table with similar properties, and why electrons in an atom can occupy the same energy level.
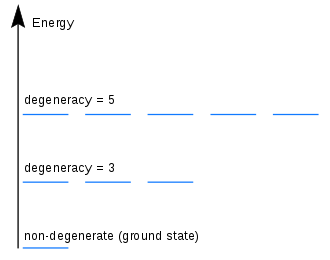
What is Degeneracy in Quantum Mechanics?
Quantum mechanics is the branch of physics that deals with the behavior of matter and energy at the atomic and subatomic level. In this field, degeneracy is the phenomenon where two or more distinct states of the same system can have the same energy. This phenomenon occurs when the system’s Hamiltonian does not depend on certain variables, such as spin or angular momentum. This article will explore the concept of degeneracy in quantum mechanics, including what it is, how it works, and its implications.
What is Degeneracy?
Degeneracy is a phenomenon that occurs in quantum mechanics where two or more distinct states of the same system can have the same energy. This occurs when the system’s Hamiltonian does not depend on certain variables, such as spin or angular momentum. The number of states that can have the same energy is known as the degree of degeneracy.
Degeneracy can be thought of as a kind of symmetry that exists in quantum mechanical systems. It is a consequence of the Heisenberg uncertainty principle, which states that a particle’s position and momentum cannot both be known simultaneously. This means that systems can exist in a variety of states, all of which have the same energy.
How Does Degeneracy Work?
Degeneracy works by allowing a system to exist in multiple quantum states, all of which have the same energy. This phenomenon occurs when the system’s Hamiltonian does not depend on certain variables, such as spin or angular momentum. As a result, the system can exist in multiple different states, all of which have the same energy.
In a degenerate system, each of the states is referred to as a “degenerate state”. These degenerate states are characterized by the fact that they all have the same energy. In addition, these states are also characterized by the fact that they cannot be distinguished from each other. This is because they all have the same energy. As a result, the system exists in a “superposition” of all of the degenerate states.
Implications of Degeneracy
The implications of degeneracy in quantum mechanics are far-reaching. One of the most significant implications is that it allows for the existence of “quantum entanglement”. This is a phenomenon where two particles are connected in such a way that they can interact with each other, even if they are separated by large distances. This phenomenon is the basis for many of the technologies we see today, such as quantum computers and quantum cryptography.
In addition, degeneracy also has implications for the behavior of particles. For example, it can lead to the phenomenon of “quantum tunneling”, where particles can move through barriers that would normally be impenetrable. This phenomenon has implications for the understanding of nuclear and chemical reactions, as well as for the development of new technologies.
Finally, degeneracy also has implications for the understanding of the structure of matter. It can be used to explain the behavior of particles on the atomic and subatomic level, as well as the behavior of large molecules. This has implications for our understanding of the universe and the laws that govern it.
Frequently Asked Questions
Degeneracy in quantum mechanics is the phenomenon where two or more distinct states in a system have the same energy. This concept is important for understanding the behavior of atoms and molecules and is widely used in many areas of physics.
What is Degeneracy in Quantum Mechanics?
Degeneracy in quantum mechanics is a phenomenon where two or more distinct states of a system have the same energy. This occurs when the system is subject to certain symmetries or interactions, such as the exchange of particles, that cause the energy of the system to remain the same, regardless of the state of the system. This concept is important for understanding the behavior of atoms and molecules, and is widely used in many areas of physics.
What are the Implications of Degeneracy in Quantum Mechanics?
The implications of degeneracy in quantum mechanics are important for understanding the behavior of atoms and molecules. When degeneracy occurs, the system is said to be in a superposition of states, which is a form of quantum entanglement. This means that the system is in multiple states at once and can be used to explain a variety of phenomena, such as the behavior of particles in a quantum superconductor. Additionally, degeneracy can be used to explain the behavior of electrons in a solid, as well as the behavior of quarks in a particle accelerator.
What is an Example of Degeneracy in Quantum Mechanics?
One example of degeneracy in quantum mechanics is the phenomenon of electron spin. Electrons can be either spin-up or spin-down, meaning they have an intrinsic angular momentum, or spin. When two electrons are in the same orbital, their spins must be opposite in order for the system to remain in a lower energy state. This is known as the Pauli exclusion principle, and it is an example of degeneracy in quantum mechanics.
What are the Applications of Degeneracy in Quantum Mechanics?
The applications of degeneracy in quantum mechanics are numerous and can be found in many fields of science. For example, degeneracy can be used to explain the behavior of electrons in a solid, such as the properties of semiconductors. Additionally, degeneracy can be used to explain the behavior of quarks in a particle accelerator, and is also important for understanding the behavior of atoms and molecules in chemical reactions.
What is the Difference Between Degeneracy and Symmetry?
The difference between degeneracy and symmetry is that symmetry is an inherent property of a system, while degeneracy is a consequence of symmetry. Symmetry can be broken in a system, while degeneracy is only present when certain symmetries are present. Symmetry is also a property of a system that is independent of its state, while degeneracy is only present when the system is in a certain state.

Physics 32.5 Statistical Thermodynamics (35 of 39) What is a Degenerate Quantum State?
In conclusion, the concept of degeneracy in quantum mechanics plays a crucial role in understanding the behavior of subatomic particles. The phenomenon of degeneracy arises when two or more quantum states have the same energy level, leading to the possibility of alternative paths for the particles to follow. This effect has significant implications in various areas of physics, including quantum computing, condensed matter physics, and atomic physics.
Understanding degeneracy is essential for overcoming challenges in these fields and developing new technologies that rely on quantum mechanics. As we continue to explore the mysteries of the subatomic world, it is clear that degeneracy will remain a fundamental concept in quantum mechanics. By grasping the principles behind degeneracy and its effects, scientists can unlock new opportunities for innovation and discovery, leading to significant advancements in our understanding of the universe.