Absorbance and transmittance are two critical concepts in the field of spectroscopy, which is the study of the interaction between light and matter. Spectroscopy is widely used in various scientific fields, including chemistry, physics, biology, and medicine. The relationship between absorbance and transmittance is fundamental to understanding the principles of spectroscopy and its applications.
Absorbance and transmittance are related to the amount of light that passes through a sample. Absorbance measures the amount of light that is absorbed by the sample, while transmittance measures the amount of light that passes through it. There is an inverse relationship between absorbance and transmittance: as the absorbance of a sample increases, its transmittance decreases. This relationship is described by the Beer-Lambert law, which states that the absorbance of a sample is directly proportional to its concentration and the path length of the light through the sample. Understanding the relationship between absorbance and transmittance is essential for interpreting spectroscopic data, designing experiments, and making accurate measurements in various scientific fields.
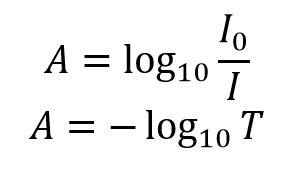
Absorbance and Transmittance
Absorbance and transmittance are two terms that are used in the field of optics to describe light and its interaction with matter. Transmittance is the amount of light that is transmitted through an object or medium, while absorbance is the amount of light that is absorbed by an object or medium. In this article, we will explore the relationship between absorbance and transmittance.
What is Absorbance?
Absorbance is a measure of the amount of light energy that is absorbed by an object or medium. It is expressed as a fraction of the total amount of light energy that is incident on the object or medium. It is usually expressed in terms of the logarithm of the ratio of light incident on the object or medium to the light that is transmitted through the object or medium.
Absorbance can be used to measure the amount of light that is absorbed by a material. This is important for understanding how light interacts with materials, as it can help to determine the degree of light absorption by a material and how that affects the properties of the material.
What is Transmittance?
Transmittance is a measure of the amount of light energy that is transmitted through an object or medium. It is expressed as a fraction of the total amount of light energy that is incident on the object or medium. Transmittance is usually expressed in terms of the logarithm of the ratio of light transmitted through the object or medium to the light that is incident on the object or medium.
Transmittance is important for understanding how light interacts with materials, as it can help to determine the degree of light transmission through a material and how that affects the properties of the material.
The Relation Between Absorbance and Transmittance
The relation between absorbance and transmittance is that the amount of light that is absorbed by a material is equal to the amount of light that is transmitted through the material. This means that, if a certain amount of light is incident on a material, the amount of light that is absorbed by the material is equal to the amount of light that is transmitted through the material.
This is because, when light interacts with a material, some of the light is absorbed into the material, while some of the light passes through the material. The amount of light that is absorbed and transmitted depends on the properties of the material, such as its optical characteristics and the amount of light that is incident on the material.
In addition, the relation between absorbance and transmittance can also be used to calculate the optical properties of a material, such as its refractive index, absorption coefficient, and scattering coefficient. By measuring the absorbance and transmittance of a material, the optical properties of the material can be determined. This can be used to design optical components and systems, such as lenses and optical fibers.
Frequently Asked Questions
Absorbance and transmittance are related to the light that passes through a sample. Here are a few commonly asked questions about this topic.
What is the relation between absorbance and transmittance?
Absorbance and transmittance are both measures of the amount of light that passes through a sample. Absorbance is the measure of how much light is absorbed by the sample, and transmittance is the measure of how much light is transmitted through the sample. The absorbance is equal to the logarithm of the reciprocal of the transmittance. In other words, if the absorbance is known, the transmittance can be calculated and vice versa.
What is the formula for calculating absorbance?
The formula for calculating absorbance is A = -log10(T), where A is the absorbance and T is the transmittance. This equation means that the absorbance is equal to the negative logarithm of the reciprocal of the transmittance.
What is the formula for calculating transmittance?
The formula for calculating transmittance is T = 10-A, where T is the transmittance and A is the absorbance. This equation means that the transmittance is equal to the ten to the power of minus the absorbance.
What is the range for absorbance and transmittance?
The range for absorbance is 0 to infinity, and the range for transmittance is 0 to 1. Generally, the higher the absorbance, the lower the transmittance and vice versa.
What is the difference between absorbance and extinction?
Absorbance and extinction are both measures of how much light is absorbed by a sample. The difference between the two is that absorbance measures the amount of light absorbed by the sample, while extinction measures the amount of light scattered by the sample.
In conclusion, the relationship between absorbance and transmittance is a fundamental concept in the field of spectroscopy. Absorbance and transmittance are inversely related, meaning that as the absorbance of a substance increases, its transmittance decreases. This relationship is crucial in determining the concentration of a substance in a solution, as the amount of light absorbed by the substance depends on the concentration of the substance in the solution. Therefore, by measuring the absorbance or transmittance of a substance, scientists can calculate its concentration accurately.
Furthermore, the relationship between absorbance and transmittance is not only important in scientific research, but it also has practical applications in various industries. For instance, in the food industry, spectrophotometry is used to detect impurities in food products. Similarly, in the pharmaceutical industry, spectrophotometry is used to measure the amount of active ingredient in a drug. As such, understanding the relationship between absorbance and transmittance plays a crucial role in ensuring the safety and efficacy of the products we consume. Overall, the relationship between absorbance and transmittance is a crucial concept in the field of spectroscopy, with practical applications in various industries.




qui minus aperiam quo molestiae quo est fugit labore incidunt voluptate amet laboriosam labore. omnis dolorum in velit omnis aut in officia qui autem magni blanditiis labore. unde laborum eos repudian
great site
non dolores sed harum corrupti corrupti aut corrupti et ipsum quaerat odio neque voluptas molestiae nostrum laborum. at officia nihil maiores laudantium temporibus voluptate non sint. nihil corrupti o