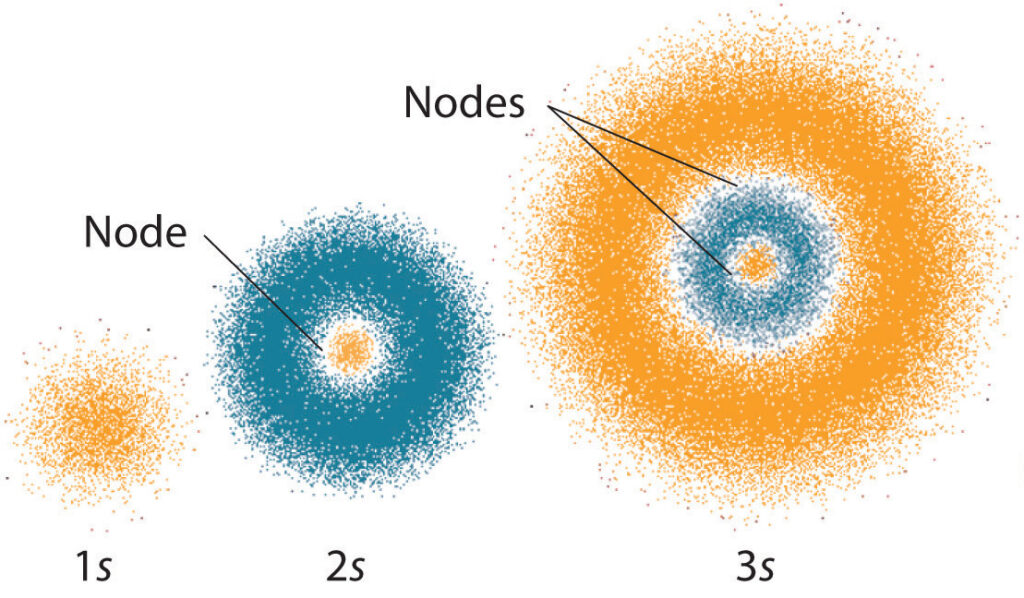
Quantum numbers are essential in describing the behavior of subatomic particles, such as electrons. These numbers define the location, energy, and spin of electrons in an atom, and their combination determines the unique identity of each electron. As a professional writer, I can tell you that understanding quantum numbers is crucial in grasping the fundamental principles of quantum mechanics.
A set of valid quantum numbers consists of four parameters – principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number. Each parameter has a unique value that determines the electron’s position and behavior in an atom. These quantum numbers are interrelated and are used to describe the electronic configuration of atoms, which is essential in predicting chemical behavior. Without the concept of quantum numbers, the structure and behavior of atoms would remain a mystery, and many scientific and technological advancements would not have been possible.
The principal quantum number (n) describes the energy level of an electron. The angular momentum quantum number (l) describes the shape of the electron orbital. The magnetic quantum number (mℓ) describes the orientation of the electron orbital in space. Finally, the spin quantum number (s) describes the spin of the electron. These four quantum numbers together form a set of valid quantum numbers.

Which are the valid quantum numbers?
Quantum numbers are a set of four numbers used by physicists and chemists to describe the energy and angular momentum of an electron in an atom. They are integral to the understanding of atomic structure and the behavior of molecules. Each quantum number describes a different property of the electron, and together they can be used to determine the energy and angular momentum of the electron.
Principal Quantum Number (n)
The principal quantum number (n) describes the size and energy of the orbital that the electron is in. The larger the value of n, the higher the energy of the orbital and the further the electron is from the nucleus. The principal quantum number can range from 1 to infinity.
The principal quantum number also affects the angular momentum of the orbital. As the value of n increases, the angular momentum of the orbital increases. This is because the larger the size of the orbital, the more space there is for the electron to move around.
Azimuthal Quantum Number (l)
The azimuthal quantum number (l) describes the shape of the orbital the electron is in. The azimuthal quantum number can range from 0 to (n – 1). The value of l determines the number of subshells in an orbital, with each subshell having a different energy level.
The azimuthal quantum number also affects the angular momentum of the orbital. As the value of l increases, the angular momentum of the orbital increases. This is because the more complex the shape of the orbital, the more space there is for the electron to move around.
Magnetic Quantum Number (ml)
The magnetic quantum number (ml) describes the orientation of the orbital in space. The magnetic quantum number can range from -l to +l. The value of ml determines the number of orbitals in a subshell, with each orbital having a different energy level.
The magnetic quantum number also affects the angular momentum of the orbital. As the value of ml increases, the angular momentum of the orbital increases. This is because the more complex the orientation of the orbital, the more space there is for the electron to move around.
Spin Quantum Number (ms)
The spin quantum number (ms) describes the spin of the electron. The spin quantum number can have a value of +1/2 or -1/2. This is the only quantum number that does not affect the angular momentum of the orbital.
The spin quantum number is important because it determines the total angular momentum of the electron. If the spin quantum number is +1/2, then the total angular momentum is equal to the orbital angular momentum. If the spin quantum number is -1/2, then the total angular momentum is equal to the negative of the orbital angular momentum.
Frequently Asked Questions About Quantum Numbers
Quantum numbers are mathematical expressions that describe the state of the quantum mechanical system. They are used to describe the energy and angular momentum of particles and determine the structural and spectroscopic properties of atoms and molecules.
What Are Quantum Numbers?
Quantum numbers are mathematical expressions that describe the state of a system in quantum mechanics. They are used to describe the energy and angular momentum of particles, and are often used to determine the structural and spectroscopic properties of atoms and molecules. Quantum numbers can be used to identify the different energy levels of electrons in an atom, which in turn can be used to calculate the structure of the atom and predict its behavior.
What Are the Different Types of Quantum Numbers?
There are four primary types of quantum numbers: the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (m_l), and the spin quantum number (m_s). The principal quantum number (n) is used to describe the energy levels of the electrons in an atom. The angular momentum quantum number (l) describes the shape of the orbital that the electrons occupy, and the magnetic quantum number (m_l) describes the orientation of the orbital. Lastly, the spin quantum number (m_s) describes the spin of the electron.
What Is the Significance of Quantum Numbers?
Quantum numbers are important because they are used to describe the behavior of atoms and molecules. By understanding the quantum numbers of a system, scientists can determine the structure of the atom and its spectroscopic properties. Furthermore, understanding the quantum numbers of a system can be used to predict its behavior and the physical properties of the particles involved.
How Are Quantum Numbers Used?
Quantum numbers are used to describe the energy and angular momentum of particles and can be used to calculate the structure of atoms and molecules. They are also used to predict the behavior of the system and identify the physical properties of the particles involved. Additionally, quantum numbers can be used to determine the structure of a molecule and the spectroscopic properties of atoms.
What Is the Relationship Between Quantum Numbers and the Periodic Table?
The periodic table is created by ordering elements based on their atomic number. The atomic number of an element is determined by the number of protons in the nucleus of the atom. Each element’s atomic number is related to its electronic structure and can be used to determine the quantum numbers of the electrons in that element. Therefore, the periodic table is closely related to quantum numbers.
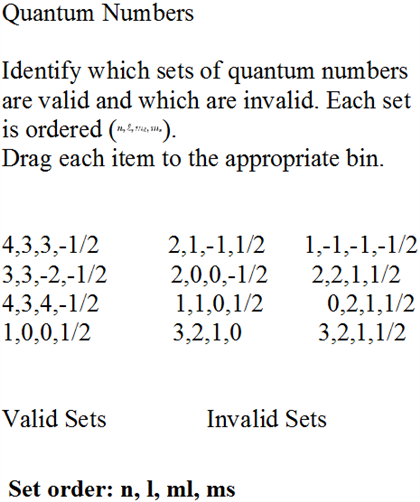
In conclusion, understanding the concept of quantum numbers is crucial to comprehend the behavior of subatomic particles. A set of valid quantum numbers helps to determine the energy levels, the spatial orientation, and the spin of an electron. These numbers are integral to the fundamental principles of chemistry and physics, and their significance cannot be overstated.
As scientists continue to explore the mysteries of the quantum world, the importance of quantum numbers will only become more apparent. With new discoveries and technological advancements, we can expect to gain a deeper understanding of the behavior of subatomic particles and the role that quantum numbers play in their interactions. In short, a set of valid quantum numbers is a powerful tool that can help scientists unlock the secrets of the universe, and its significance cannot be overstated.