The atom, the building block of all matter, has been the subject of scientific inquiry for centuries. As technology advanced, scientists developed new models to better understand the structure and behavior of atoms. One such model is the quantum mechanical model, which is based on the principles of quantum mechanics.
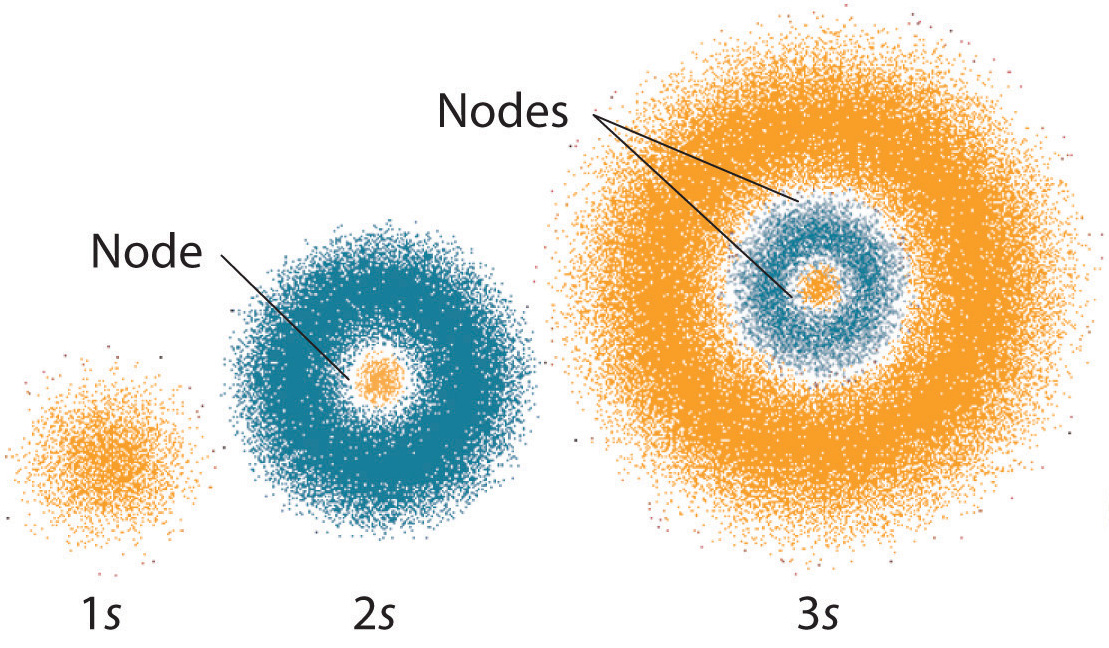
The quantum mechanical model of the atom is a mathematical representation that explains the behavior of electrons in an atom. Unlike the classical model, which depicts electrons as orbiting the nucleus in fixed paths, the quantum mechanical model suggests that electrons exist in a cloud-like region around the nucleus, known as an atomic orbital. This revolutionary model has greatly contributed to our understanding of atomic structure and has helped scientists make groundbreaking discoveries in the field of chemistry and physics.
The quantum mechanical model of the atom is a model of the atom that uses quantum mechanics to describe its structure and behavior. It is based on the idea that electrons move around the nucleus in specific energy levels, or orbitals. This model predicts the energies of the electrons in the atom and the probability of finding the electrons at any given location.
What is the Quantum Mechanical Model of the Atom?
The Quantum Mechanical Model of the atom is a model that explains the behavior of an atom using quantum mechanics. It is based on the principle that an atom is composed of subatomic particles, such as electrons, protons and neutrons, that are held together by a strong force, the nuclear force. This model is used to explain the structure of atoms, how they interact with each other, and how they interact with light.
History of Quantum Mechanical Model
The Quantum Mechanical Model of the atom was first proposed in the early 1900s by Danish physicist Niels Bohr. Bohr’s model was based on the observation that electrons in an atom can only occupy certain energy levels. He proposed that the electrons in an atom are arranged in shells, and that the energy levels within these shells are determined by the interaction between the electron and the nucleus. This model was later refined by other scientists, such as Wolfgang Pauli and Erwin Schrödinger, who developed the mathematical equations that describe the behavior of electrons in an atom.
Fundamental Principles of the Quantum Mechanical Model
The Quantum Mechanical Model of the atom is based on several fundamental principles. The first is the Heisenberg Uncertainty Principle, which states that it is impossible to know both the position and momentum of a particle at the same time. This principle has important implications for the behavior of electrons in an atom, as it means that electrons can be found in many different positions at the same time.
The second principle is the Pauli Exclusion Principle, which states that two electrons cannot occupy the same energy level in an atom. This principle explains why electrons in an atom are arranged in shells and why they do not collapse into the nucleus. Finally, the Schrodinger Equation is used to calculate the behavior of electrons in an atom, and it is based on the principles of quantum mechanics.
Frequently Asked Questions About the Quantum Mechanical Model of the Atom
Quantum mechanics is a branch of physics that focuses on the behavior of matter and energy at the atomic and subatomic level. It is the basis for the quantum mechanical model of the atom, which describes an atom as a system of particles with specific properties. This model is used to explain the structure of atoms and their interactions with other atoms and particles.
What is the Quantum Mechanical Model of the Atom?
The quantum mechanical model of the atom is a theoretical model that describes how atoms interact with each other and other particles. It is based on quantum mechanics, which is a branch of physics that focuses on the behavior of matter and energy at the atomic and subatomic level. This model describes an atom as a system of particles that have specific properties and that interact with each other in a specific way. The model also describes how electrons move around in an atom, how the nucleus of an atom is composed, and how electrons interact with the nucleus.
What Are the Main Features of the Quantum Mechanical Model of the Atom?
The main features of the quantum mechanical model of the atom are that it describes an atom as a system of particles in which the electrons move around in certain orbits. The electrons interact with the nucleus of the atom and with each other. The model also describes the energy levels of electrons and the way they interact with light and other particles.
The model also explains how electrons are arranged in an atom and how they interact with other particles. It also explains the structure of the nucleus and how it is held together. The model also describes the behavior of the electrons in an atom and how they interact with each other and with the nucleus.
What Are the Limitations of the Quantum Mechanical Model of the Atom?
The main limitation of the quantum mechanical model of the atom is that it does not account for all of the properties of atoms. For example, the model does not explain the properties of the nucleus or the behavior of the electrons at very small distances. It also does not explain the behavior of matter at very high energies or temperatures.
Another limitation of the quantum mechanical model of the atom is that it does not account for all of the interactions between particles. For example, the model does not explain the behavior of particles at very small distances or explain the behavior of particles when they interact with other particles.
How Is the Quantum Mechanical Model of the Atom Used?
The quantum mechanical model of the atom is used to explain the structure of atoms and their interactions with other atoms and particles. It is also used to explain the behavior of electrons in an atom and how they interact with each other and with the nucleus. The model is also used to explain the behavior of particles at very small distances and how they interact with each other and with the nucleus.
The quantum mechanical model of the atom is also used to explain the behavior of matter and energy at the atomic and subatomic level. It is also used to explain the structure of the nucleus and how it is held together. Finally, the model is used to explain the behavior of particles at very high energies and temperatures.
What Are the Advantages of the Quantum Mechanical Model of the Atom?
The main advantage of the quantum mechanical model of the atom is that it provides a framework for understanding the behavior of matter and energy at the atomic and subatomic level. This model is also useful for understanding the behavior of particles at very small distances. Furthermore, the model is also useful for understanding the behavior of particles when they interact with other particles.
Another advantage of the quantum mechanical model of the atom is that it provides a way of understanding the structure of the nucleus and how it is held together. Finally, the model is also useful for understanding the behavior of matter and energy at very high energies and temperatures.

In conclusion, the quantum mechanical model of the atom is a remarkable achievement that has helped us understand the nature of atoms and their behavior. This model has revolutionized the way we think about the subatomic world, and its predictions have been rigorously tested and verified. The model has provided us with a powerful tool to explain and predict the behavior of atoms, which has had a significant impact on fields ranging from chemistry to electronics.
The quantum mechanical model has not only deepened our understanding of the atom but also opened up new avenues of research and discovery. It has helped us explore the fundamental nature of matter and energy, and has led to the development of new technologies that have transformed our world. As we continue to explore the mysteries of the quantum world, the quantum mechanical model will undoubtedly play a vital role in shaping our understanding of the universe and the physical laws that govern it.