Quantum mechanics is a fascinating branch of physics that seeks to explain the behavior of matter and energy at the atomic and subatomic levels. One of the most important concepts in quantum mechanics is the idea of quantum numbers. These numbers are used to describe the properties of subatomic particles, such as their energy levels, spin, and angular momentum. However, there is a common question that arises when discussing quantum numbers: can they be negative?
At first glance, it might seem like a simple yes or no answer to this question. However, the reality is that the answer is not so straightforward. To understand why, we need to delve deeper into the world of quantum mechanics and explore the nature of quantum numbers. In this article, we will explore the concept of quantum numbers and examine whether or not they can be negative. Whether you are a physics enthusiast, a student, or simply curious about the mysteries of the universe, this article will provide you with a fascinating look into the world of quantum mechanics.
Yes, l can be negative in quantum numbers. The absolute value of l is known as the orbital angular momentum quantum number. This determines the shape of an electron’s orbital around the nucleus and can have a value between 0 and (n-1). It can also have negative values.
Can l be Negative in Quantum Numbers?
Quantum numbers are used to describe the properties of an atom’s electrons and other subatomic particles. They are an integral part of understanding the behavior of atoms and molecules and the laws of quantum mechanics. One of the most important questions that arise when discussing quantum numbers is whether or not it is possible for the quantum number l to be negative.
What are Quantum Numbers?
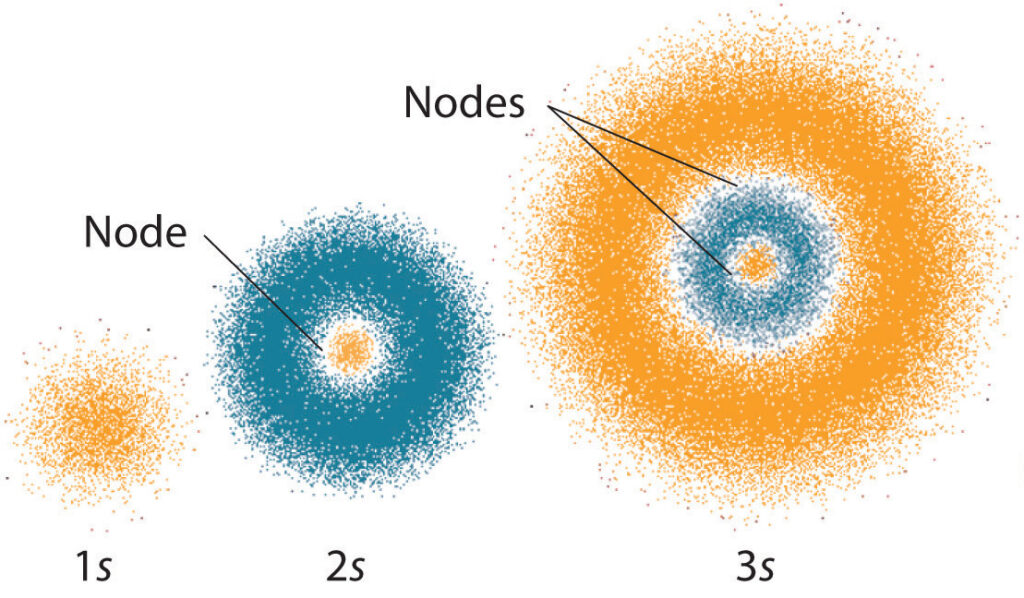
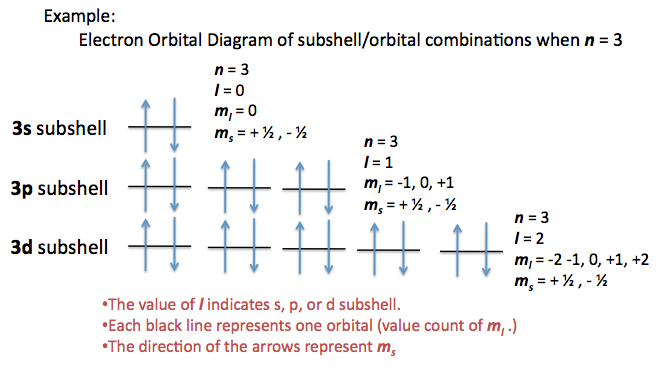
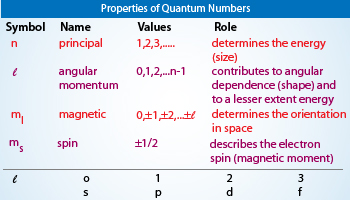
Quantum numbers are a set of numbers that describe the energy levels, angular momentum, and other properties of a particle. Each number corresponds to a characteristic of the particle and can be used to describe its behavior. The four main quantum numbers are the principal quantum number (n), angular momentum quantum number (l), magnetic quantum number (m_l), and spin quantum number (m_s).
The principal quantum number (n) describes the energy level of an electron. It can have any positive integer value from 1 to infinity. The angular momentum quantum number (l) describes the shape of an electron’s orbital. It can have any integer value from 0 to (n-1). The magnetic quantum number (m_l) describes the orientation of an electron’s orbital. It can have any integer value from -l to +l. The spin quantum number (m_s) describes the spin of an electron. It can have either a +1/2 or -1/2 value.
Can l be Negative?
The angular momentum quantum number (l) is the only quantum number that can have a negative value. It can have a value of any integer from 0 to (n-1), including negative integers. However, the absolute value of l must always be less than or equal to the principal quantum number (n).
For example, if the principal quantum number (n) is 2, the angular momentum quantum number (l) can be either 0 or 1. If the principal quantum number (n) is 3, the angular momentum quantum number (l) can be either 0, 1, or 2. If the principal quantum number (n) is 4, the angular momentum quantum number (l) can be either 0, 1, 2, or 3.
It is also important to note that the magnetic quantum number (m_l) must always be within the range of -l to +l. This means that if the angular momentum quantum number (l) is negative, the magnetic quantum number (m_l) must also be negative.
Conclusion
In conclusion, the angular momentum quantum number (l) is the only quantum number that can have a negative value. The absolute value of l must always be less than or equal to the principal quantum number (n). Additionally, if the angular momentum quantum number (l) is negative, the magnetic quantum number (m_l) must also be negative. Understanding the behavior of quantum numbers is an important part of understanding the behavior of atoms and molecules.
Frequently Asked Questions About Can l be Negative in Quantum Numbers?
Quantum numbers are a set of four numbers used to describe the energy states of an electron in an atom. They are important in understanding how atoms and molecules interact with each other and with the environment. This article will answer the question of whether or not a quantum number can be negative.
Can a Quantum Number Be Negative?
The answer to this question is yes, quantum numbers can be negative. This is because the quantum numbers are related to the energy of the electrons in an atom. When an electron is in an excited state, it has more energy than when it is in a ground state. Therefore, when the electron is in an excited state, the quantum numbers associated with it may be negative.
When an electron is in a ground state, the quantum numbers associated with it are usually positive. This is because the energy of the electrons in the ground state is lower than that of the electrons in the excited state. Therefore, the quantum numbers are positive because they are associated with a lower energy level.
What Is the Significance of Having Negative Quantum Numbers?
Negative quantum numbers can be important in understanding how atoms interact with each other and with their environment. For example, when an electron is in an excited state, it can interact with other electrons in the environment and cause them to become excited as well. By understanding the energy levels associated with the quantum numbers, scientists can better understand how these interactions occur.
In addition, negative quantum numbers can help scientists understand the structure of molecules and how they interact with each other. By understanding the energy levels associated with the quantum numbers, scientists can better understand the structure of molecules and how they interact with each other. This can help in understanding the properties of molecules and how they interact with their environment.
What Are the Four Quantum Numbers?
The four quantum numbers are the principal quantum number, the angular momentum quantum number, the magnetic quantum number, and the spin quantum number. The principal quantum number is a number that describes the energy level of an electron in an atom. The angular momentum quantum number is a number that describes the angular momentum of an electron in an atom. The magnetic quantum number is a number that describes the orientation of an electron in an atom. The spin quantum number is a number that describes the spin of an electron in an atom.
How Are the Quantum Numbers Related to Electrons?
The quantum numbers are related to the energy levels of electrons in an atom. The principal quantum number is related to the energy level of the electron. The angular momentum quantum number is related to the angular momentum of the electron. The magnetic quantum number is related to the orientation of the electron. The spin quantum number is related to the spin of the electron.
By understanding these relationships, scientists can better understand how electrons interact with their environment and other particles. This can help in understanding the structure of molecules and how they interact with their environment.
What Are the Possible Values of the Quantum Numbers?
The possible values of the quantum numbers depend on the type of atom they are associated with. For example, the principal quantum number can range from 1 to 7, the angular momentum quantum number can range from 0 to 5, the magnetic quantum number can range from -5 to 5, and the spin quantum number can range from -1/2 to +1/2.
The quantum numbers are important in understanding the energy states of an electron in an atom. By understanding the possible values of the quantum numbers, scientists can better understand the structure of molecules and how they interact with their environment. This can help in understanding the properties of molecules and how they interact with their environment.
How To Determine The Maximum Number of Electrons Using Allowed Quantum Numbers – 8 Cases
In conclusion, the concept of quantum numbers can be quite abstract and confusing, especially for those who are not well-versed in the field of physics. However, one thing is for certain: quantum numbers are a fundamental component of quantum mechanics and play a crucial role in understanding the behavior of subatomic particles. While it is not possible to have negative values for some quantum numbers, such as the principal quantum number, there are others, such as the magnetic quantum number, that can take on positive or negative values.
Overall, the study of quantum mechanics is a fascinating and complex field that continues to captivate scientists and researchers around the world. As our understanding of subatomic particles and their behavior continues to evolve, so too will our understanding of quantum numbers and their role in the world of physics. Whether you are an aspiring physicist, a curious student, or simply someone interested in learning more about the world around us, the study of quantum mechanics and quantum numbers is sure to offer new insights and discoveries for years to come.