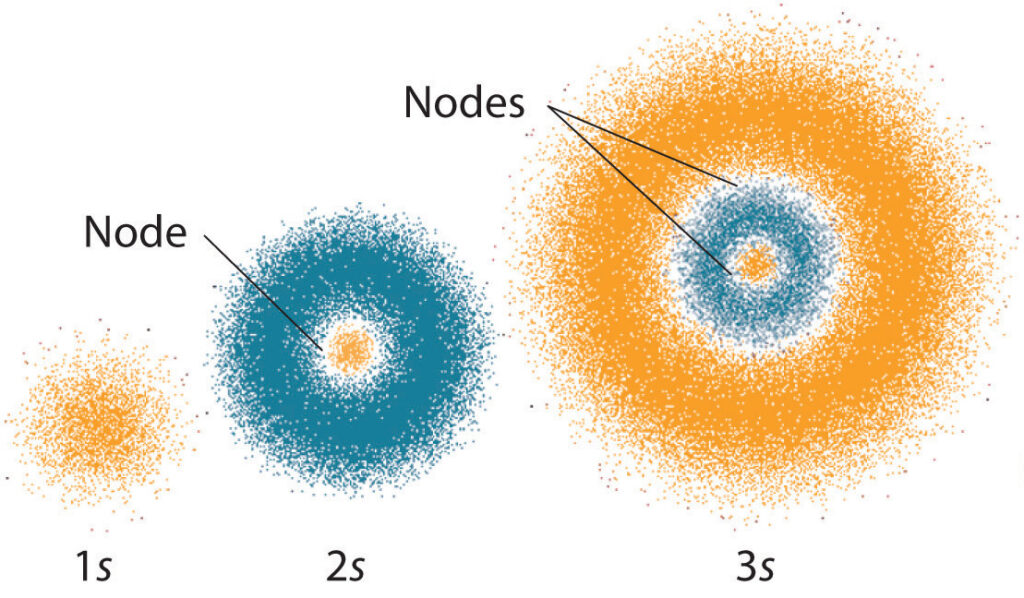
Quantum mechanics is a fascinating field of science that has revolutionized our understanding of the universe. It has provided us with a new set of rules that govern the behavior of particles at the atomic and subatomic level. One of the fundamental concepts of quantum mechanics is the set of quantum numbers, which describes the properties of electrons in an atom. The four quantum numbers are n, l, m, and s, each with a specific range of values that determine the energy, shape, orientation, and spin of an electron. However, not all combinations of these quantum numbers are valid, and this leads us to the intriguing question, which set of quantum numbers is invalid?
To answer this question, we need to understand the rules of quantum mechanics that define the allowed values of the quantum numbers. The first quantum number, n, determines the energy level of an electron and can have any positive integer value. The second quantum number, l, describes the shape of the electron’s orbital and can have values ranging from 0 to n-1. The third quantum number, m, specifies the orientation of the orbital in space and can have values ranging from -l to +l. Finally, the fourth quantum number, s, represents the spin of the electron and can have only two values, +1/2 or -1/2. Any combination of these quantum numbers that violates these rules is considered invalid. Therefore, the challenge is to identify the set of quantum numbers that does not follow these guidelines and explain why it is not valid.
The set of quantum numbers (n, l, ml, ms) n = 2, l = 2, ml = 1, ms = -2/2 is invalid. The total angular momentum quantum number l must be less than the principal quantum number n. The magnetic quantum number ml can range from -l to l and the spin quantum number ms can range from -1/2 to +1/2. The set of quantum numbers provided does not satisfy these conditions.
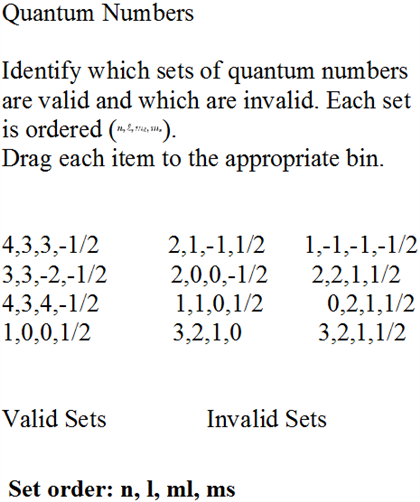
Which Set of Quantum Numbers is Invalid?
Quantum numbers are an important part of quantum mechanics and are used to describe the behavior of atoms and their components. Each set of quantum numbers consists of four different numbers that are used to calculate the energies of electrons in atoms. However, for some set of quantum numbers, the values cannot correspond to any atomic state. This article explores which set of quantum numbers are considered invalid.
What are Quantum Numbers?
Quantum numbers are mathematical descriptors used to describe the properties of electrons in an atom. They are a set of four numbers (n, l, ml and ms) that are used to identify the energy level of an electron. The first quantum number (n) is the principal quantum number and indicates the shell or energy level of the electron. The second quantum number (l) is the angular momentum quantum number and indicates the orbital shape of the electron. The third quantum number (ml) is the magnetic quantum number and indicates the orientation of the orbital in space. The fourth quantum number (ms) is the spin quantum number and indicates the spin of the electron.
Which Set of Quantum Numbers is Invalid?
In general, any set of quantum numbers that has a principal quantum number (n) of zero or a negative number is considered invalid. This is because the principal quantum number is related to the energy level of the electron, and zero or negative energies are not possible. Additionally, any set of quantum numbers with an angular momentum quantum number (l) greater than the principal quantum number, an invalid magnetic quantum number, or an invalid spin quantum number, is considered invalid.
For example, a set of quantum numbers with n=2, l=4, ml=2, and ms=1/2 is invalid because the angular momentum quantum number (l) is greater than the principal quantum number (n). Similarly, a set of quantum numbers with n=3, l=1, ml=3, and ms=1/2 is invalid because the magnetic quantum number (ml) is greater than the angular momentum quantum number (l).
Conclusion
In general, any set of quantum numbers with a principal quantum number of zero or a negative number, an angular momentum quantum number greater than the principal quantum number, an invalid magnetic quantum number, or an invalid spin quantum number, is considered invalid. Knowing which set of quantum numbers is invalid is an important part of understanding quantum mechanics.
Frequently Asked Questions
This section contains answers to questions related to an invalid set of quantum numbers.
What is a set of quantum numbers?
A set of quantum numbers is a set of numbers that describe the properties of an electron in an atom. These numbers are the principal quantum number, the angular momentum quantum number, the magnetic quantum number, and the spin quantum number. Each one of these numbers describes a different aspect of the electron’s behavior and helps to define its position and energy within an atom.
What makes a set of quantum numbers invalid?
A set of quantum numbers is considered invalid if any of the quantum numbers are outside of the accepted range for that particular type of quantum number. For example, the principal quantum number must be an integer between 1 and 7. If the number is outside of this range, then the set of quantum numbers is considered invalid.
What happens if an invalid set of quantum numbers is used?
Using an invalid set of quantum numbers can lead to inaccurate results and calculations. This is because the quantum numbers are used to define the electron’s position and energy in an atom, and if the numbers are incorrect, then the results are likely to be inaccurate.
What should be done if an invalid set of quantum numbers is used?
If an invalid set of quantum numbers is used, then the user should double-check their calculations and make sure that the quantum numbers they are using are within the accepted ranges. If they are not, then the user should try to find the correct set of quantum numbers and use them instead.
Where can I find more information about sets of quantum numbers?
More information about sets of quantum numbers can be found in textbooks, online resources, and websites dedicated to quantum mechanics. These sources will provide detailed information about the principles of quantum numbers and how to use them in calculations. Additionally, many universities offer courses dedicated to quantum mechanics that can be taken in order to learn more about the subject.
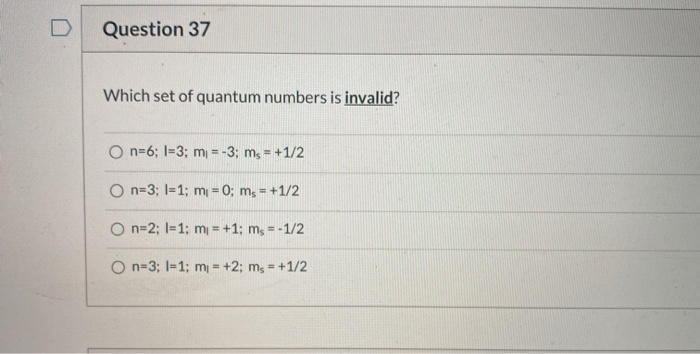
Quantum Numbers – Valid and Invalid Sets
In conclusion, understanding the concept of quantum numbers is crucial in quantum mechanics, as it helps in describing the properties of an atom, including its energy levels and electron configuration. While there are valid sets of quantum numbers that can accurately describe an atom, there are also invalid sets that may not make sense or violate the laws of quantum mechanics.
Therefore, it is important to always double-check and verify the validity of a set of quantum numbers before using them in any calculations or experiments. By doing so, scientists and researchers can ensure the accuracy and reliability of their findings, leading to better understanding and advancements in the field of quantum mechanics.