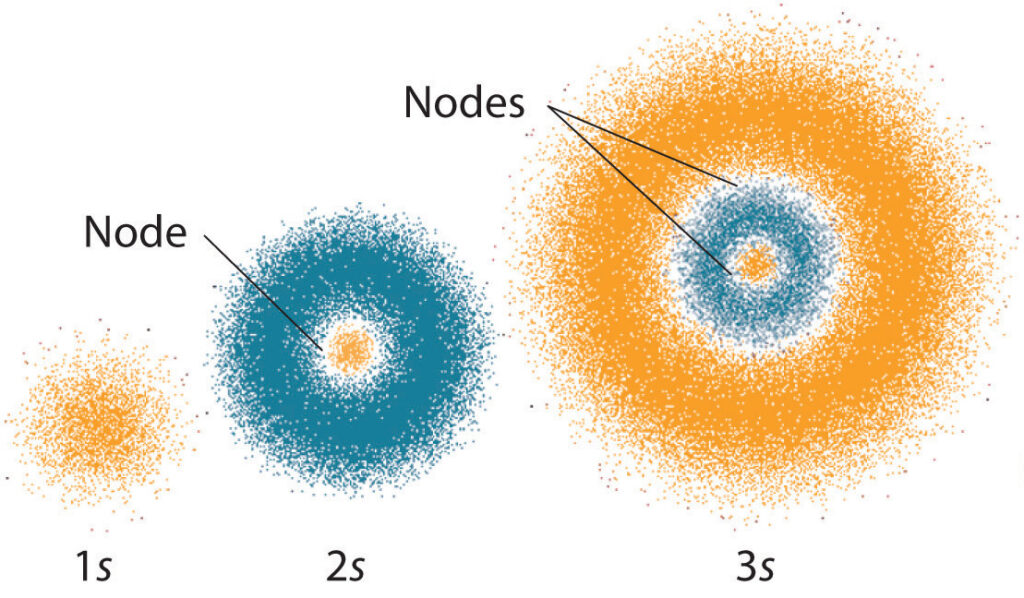
Quantum mechanics is a fascinating field of study that has revolutionized our understanding of the physical world. At the heart of this field are the quantum numbers, a set of values that describe the properties of subatomic particles. These numbers play a crucial role in determining the behavior and location of electrons within an atom. However, not all sets of quantum numbers can fully specify an orbital.
For those who may not be familiar with the term, an orbital is the region of space around an atom where an electron is most likely to be found. There are four quantum numbers that can be used to describe the properties of an electron and its associated orbital. But which set of quantum numbers cannot fully specify an orbital? This is a question that has puzzled scientists for decades, and the answer lies in the intricacies of quantum mechanics. In this article, we will explore this fascinating topic in detail and shed light on the mysterious world of quantum mechanics.
No set of quantum numbers can specify an orbital since each quantum number only specifies a particular property of the orbital. Quantum numbers describe the state of an electron within an atom and are used to differentiate between electrons in different orbitals. The four quantum numbers are the principal quantum number (n), angular momentum quantum number (l), magnetic quantum number (m_l), and spin quantum number (m_s).

Which Set of Quantum Numbers Cannot Specify an Orbital?
Quantum numbers are used to describe the unique characteristics of an electron in an atom. They provide information about the electron’s energy level, angular momentum, and orientation in space. However, not all quantum numbers are capable of specifying an orbital. In this article, we will discuss which set of quantum numbers cannot specify an orbital.
What Are Quantum Numbers?
Quantum numbers are numerical values used to describe the properties of electrons in an atom. They are based on the quantum mechanical model of the atom, which states that electrons occupy specific energy levels around the nucleus. Each electron has a unique set of quantum numbers that can be used to identify it. These numbers are usually represented by the letters n, l, m, and s.
The first quantum number, n, is known as the principal quantum number and is used to describe the energy level of the electron. The second quantum number, l, is known as the angular momentum quantum number and is used to describe the electron’s orbital shape. The third quantum number, m, is known as the magnetic quantum number and is used to describe the orientation of the electron in space. The fourth quantum number, s, is known as the spin quantum number and is used to describe the electron’s spin.
Which Set of Quantum Numbers Cannot Specify an Orbital?
The spin quantum number, s, cannot be used to specify an orbital. This is because the spin quantum number only describes the electron’s spin, not its location in space. Therefore, it cannot be used to determine the electron’s position in an orbital.
The other three quantum numbers, n, l, and m, can be used to describe an electron’s location in an orbital. The principal quantum number, n, is used to indicate the energy level of the electron. The angular momentum quantum number, l, is used to indicate the orbital shape. And the magnetic quantum number, m, is used to indicate the orientation of the electron in space.
Conclusion
In conclusion, the spin quantum number, s, cannot be used to specify an orbital. The other three quantum numbers, n, l, and m, can be used to describe an electron’s location in an orbital. Understanding these quantum numbers is essential for understanding the structure of atoms and molecules.
Frequently Asked Questions (FAQs) about Quantum Numbers
Quantum numbers can be used to describe the energy, shape, orientation, and size of an orbital. This article will answer some of the most commonly asked questions about quantum numbers and explain why some sets of quantum numbers cannot be used to specify an orbital.
What Are Quantum Numbers?
Quantum numbers are a set of four numbers that are used to describe the properties and behavior of electrons in an atom. They are used to describe the energy, shape, size and orientation of an orbital. The four quantum numbers are: the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (mℓ), and the spin quantum number (m_s).
Why Do Some Sets of Quantum Numbers Not Specify an Orbital?
The quantum numbers must satisfy certain conditions in order to specify an orbital. The principal quantum number (n) must be a positive integer, the angular momentum quantum number (l) must be less than the value of n, the magnetic quantum number (mℓ) must be between -l and +l, and the spin quantum number (m_s) must be either +1/2 or -1/2. If any of these conditions are not met, then the quantum numbers will not be able to specify an orbital.
What Is the Relationship Between the Quantum Numbers and the Wave Function?
The wave function of an orbital is determined by the quantum numbers. The wave function describes the probability of finding the electron in a certain location. The wave function is a mathematical equation that is used to calculate these probabilities. The quantum numbers describe the properties and behavior of the electron and are used to calculate the wave function.
How Are Quantum Numbers Used in Chemistry?
In chemistry, quantum numbers are used to describe the behavior of electrons in atoms. They are used to determine the energy, shape, size, and orientation of an orbital. Quantum numbers can also be used to determine the number of electrons in an atom and the chemical properties of an element.
What Is an Example of a Quantum Number?
An example of a quantum number is the angular momentum quantum number (l). This quantum number describes the shape of an orbital. It can take on values from 0 to n-1. For example, if the principal quantum number (n) is 3, then the angular momentum quantum number (l) can take on values of 0, 1, and 2.
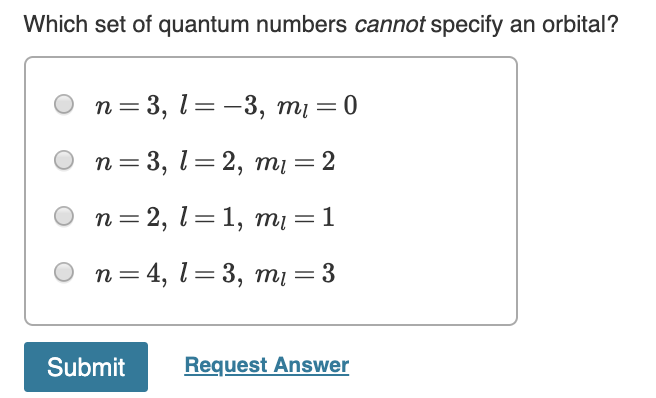
In conclusion, understanding the concept of quantum numbers is essential in grasping the behavior of atoms and their electrons. While there are four sets of quantum numbers, not all of them can specify an orbital. Specifically, the set of quantum numbers that cannot specify an orbital is the set of magnetic quantum numbers.
In summary, the magnetic quantum number, along with the other quantum numbers, is crucial in describing the behavior and properties of an atom. By understanding the limitations of each set of quantum numbers, we can better comprehend the complex nature of subatomic particles and their movements. As we continue to delve deeper into the world of quantum mechanics and atomic structure, the importance of quantum numbers cannot be overstated.