As we dive deeper into the fundamental structure of matter, we begin to encounter the mind-bending world of quantum mechanics. One of the most significant developments in this field was the creation of the quantum mechanical model of the atom. This model not only improved our understanding of the behavior of electrons within atoms but also paved the way for the development of modern technology.
The quantum mechanical model of the atom is a highly complex and abstract concept, but at its core, it describes the behavior of electrons based on their wave-like properties. This model replaced the earlier Bohr model, which had limitations in explaining the behavior of atoms beyond hydrogen. With the quantum mechanical model, scientists were able to more accurately predict the location and energy levels of electrons within atoms, leading to a better understanding of chemical reactions and the creation of new materials.
The Quantum Mechanical Model of an atom is a scientific model that explains the structure and behavior of an atom. It states that an atom is made up of a nucleus surrounded by electrons that orbit the nucleus in various energy levels. The electrons can move between the energy levels, but not to any distance from the nucleus. The model also states that an atom is composed of quarks and gluons, which interact to form the nucleus.
What is a Quantum Mechanical Model of an Atom?
The quantum mechanical model of an atom is a model of an atom that explains the behavior of its electrons and nucleus using quantum mechanics. It is based on the idea that an atom is made up of a nucleus surrounded by a cloud of electrons. The quantum mechanical model of the atom is used to describe the behavior of atoms in a variety of different processes, such as chemical reactions, bonding, and the formation of molecules.
The Nucleus
The nucleus of an atom is made up of protons and neutrons, and is held together by a strong force. The protons have a positive charge, and the neutrons have no charge. The nucleus is the smallest part of an atom, and the protons and neutrons are held together by the strong nuclear force. This force is so strong that it is not affected by the electrons that orbit the nucleus.
The protons and neutrons are arranged in a pattern called the nuclear configuration. This configuration determines the chemical behavior of the atom and is determined by the number of protons and neutrons in the nucleus. The number of protons in an atom determines its atomic number, which is used to classify the elements on the periodic table.
The Electron Cloud
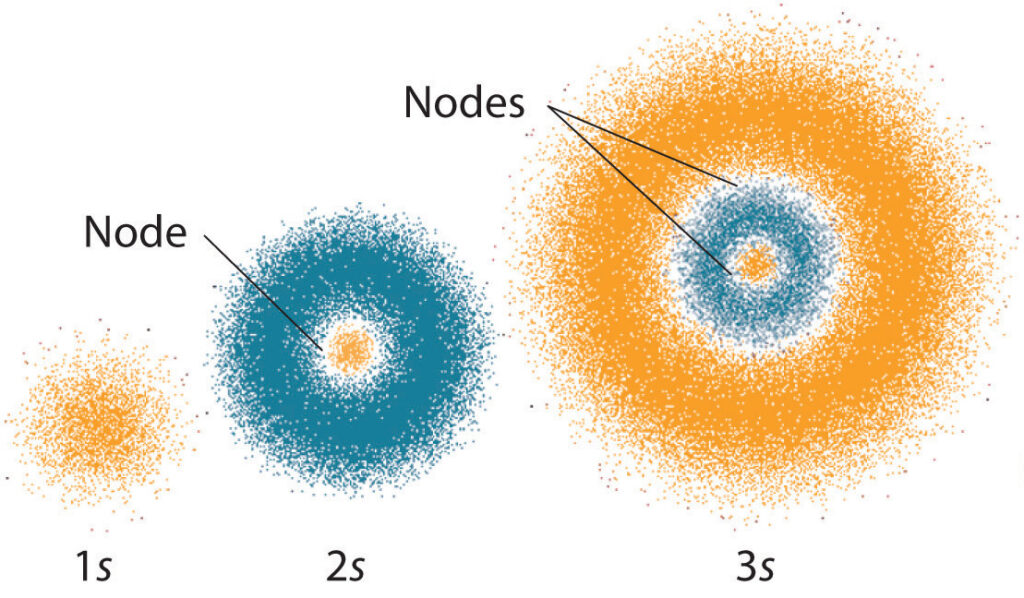
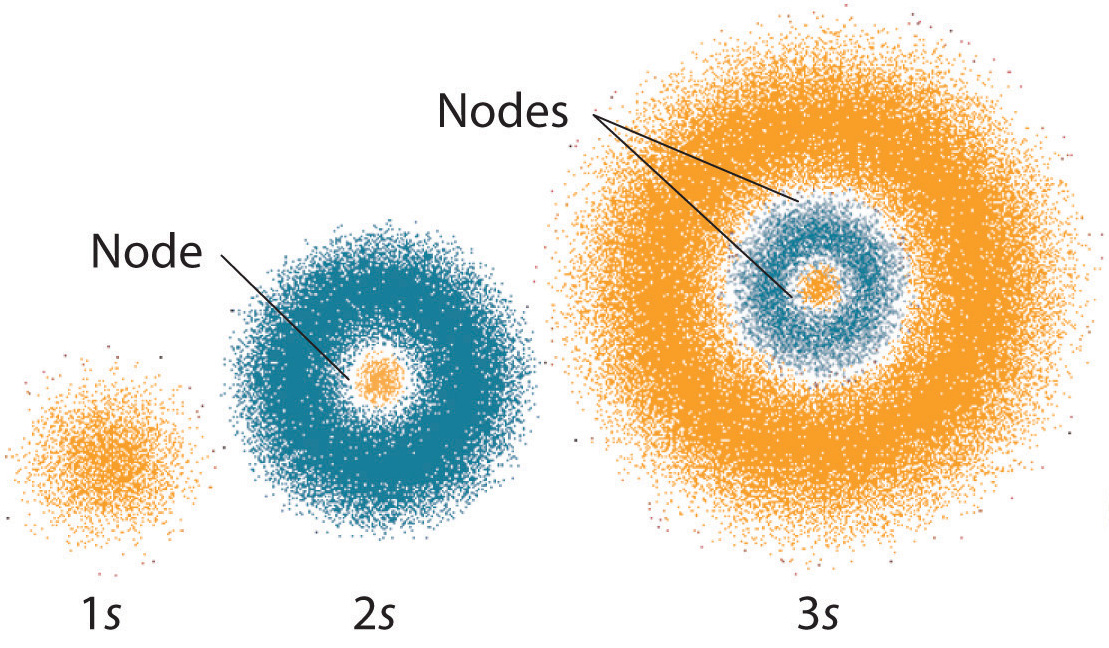
The electrons in an atom are arranged in a spherical cloud around the nucleus. This cloud is called the electron cloud. The electrons are held in the cloud by the electrostatic force, which is the force that holds the electrons and protons together. This force is weaker than the nuclear force, so the electrons can move more freely in the electron cloud.
The electrons in the electron cloud are arranged in energy levels. Each energy level is further divided into sub-levels, which are further divided into orbitals. Each orbital has a specific number of electrons, which determines the chemical behavior of the atom. The electrons in the electron cloud can move from one energy level to another, and this is how an atom can form bonds with other atoms.
Quantum Mechanics
Quantum mechanics is a branch of physics that deals with the behavior of particles on the atomic and subatomic scale. The principles of quantum mechanics are used to explain the behavior of atoms and electrons in the quantum mechanical model of an atom. The behavior of electrons in the electron cloud is determined by the Schrödinger equation, which is used to calculate the probability of an electron being in a certain location in the electron cloud.
The behavior of the nucleus is determined by the Heisenberg uncertainty principle, which states that the position and momentum of a particle can never be determined simultaneously. This means that the exact location of the nucleus cannot be known. The Heisenberg uncertainty principle also states that the energy levels of the electrons in the electron cloud can never be determined precisely.
Applications of the Quantum Mechanical Model of an Atom
The quantum mechanical model of an atom is used in many different fields, including chemistry, physics, and materials science. It is used to explain the behavior of atoms in chemical reactions, bonding, and the formation of molecules. It is also used to study the properties of materials, such as superconductors, semiconductors, and nanomaterials.
The quantum mechanical model of an atom has also been used to develop new technologies, such as quantum computers and quantum encryption. The principles of quantum mechanics are also used in medical imaging, such as X-ray imaging and MRI. These techniques allow doctors to see inside the human body and diagnose diseases.
Frequently Asked Questions
The quantum mechanical model of the atom is a physical model of the atom that is based on quantum mechanics. It is used to explain the structure and behavior of atoms, and is an important part of modern physics.
What is the quantum mechanical model of the atom?
The quantum mechanical model of the atom is a physical model of the atom that is based on quantum mechanics. This model explains the structure and behavior of atoms, and is an important part of modern physics. The model works by using the principles of quantum mechanics to describe the behavior of electrons and other subatomic particles that make up an atom. This model is used to explain the behavior of atoms in a variety of situations, including chemical reactions, radiation, and the formation of molecules.
The quantum mechanical model of the atom also describes how electrons interact with each other, and how they form bonds between atoms. This allows scientists to understand the behavior of molecules and other materials, and to create new materials with desirable properties. The model is also used to explain the properties of light, and how light interacts with matter.
How does the quantum mechanical model of the atom work?
The quantum mechanical model of the atom works by using the principles of quantum mechanics to describe the behavior of electrons and other subatomic particles that make up an atom. This includes using the Heisenberg uncertainty principle to show how the position and momentum of an electron can never be known exactly at the same time. This means that the behavior of electrons cannot be predicted with perfect accuracy, and must be described using probability.
The quantum mechanical model of the atom also takes into account the wave-particle duality of electrons. This means that electrons can behave as both particles and waves, and this duality affects the behavior of the electrons in an atom. This is used to explain the behavior of electrons in chemical bonds, the formation of molecules, and the behavior of light. The quantum mechanical model of the atom is also used to calculate the energy levels of electrons in an atom, and this can be used to explain the properties of materials.
What is the importance of the quantum mechanical model of the atom?
The quantum mechanical model of the atom is an important part of modern physics and is used to explain the structure and behavior of atoms. This model is used to explain the behavior of atoms in a variety of situations, including chemical reactions, radiation, and the formation of molecules. This model is also used to explain the properties of light, and how light interacts with matter.
The quantum mechanical model of the atom is also used to calculate the energy levels of electrons in an atom, and this can be used to explain the properties of materials. This model is also used to understand how electrons interact with each other, and how they form bonds between atoms. This allows scientists to understand the behavior of molecules and other materials, and to create new materials with desirable properties.
What are the principles of quantum mechanics?
The principles of quantum mechanics are the fundamental principles that describe the behavior of particles and energy at the atomic and subatomic level. These principles include the Heisenberg uncertainty principle, wave-particle duality, and the Schrödinger equation.
The Heisenberg uncertainty principle states that it is impossible to measure the position and momentum of a particle exactly at the same time. The wave-particle duality states that particles can behave as both waves and particles, and this affects the behavior of particles in an atom. The Schrödinger equation is a mathematical equation used to describe the behavior of particles in an atom. These principles are used to explain the behavior of atoms and other subatomic particles, and are important for understanding the quantum mechanical model of the atom.
What is the Schrödinger equation?
The Schrödinger equation is a mathematical equation used to describe the behavior of particles in an atom. This equation was developed by Austrian physicist Erwin Schrödinger in 1925, and it describes how the energy of a particle is related to its position. This equation is used to calculate the energy levels of electrons in an atom, and this can be used to explain the properties of materials.
The Schrödinger equation is also used to describe the behavior of electrons in chemical bonds, and how they interact with each other. This equation is an important part of the quantum mechanical model of the atom, and is used to explain the behavior of atoms in a variety of situations. This equation is also used to calculate the probability of an electron being in a certain position, and this is used to explain the behavior of light, and how light interacts with matter.

In conclusion, the quantum mechanical model of the atom is a highly complex and fascinating subject that provides a deeper understanding of the nature of the universe. This model has allowed scientists to make incredible advancements in the field of physics and chemistry, revolutionizing our understanding of matter and energy. Through the use of mathematical equations and advanced theories, scientists have been able to unlock the mysteries of the atom and gain a more comprehensive view of the world around us.
Furthermore, the quantum mechanical model has practical applications in fields such as medicine, engineering, and technology. It has led to the development of cutting-edge technologies such as quantum computing, which has the potential to revolutionize the way we process information. As our understanding of this model continues to grow, we can expect to see even more breakthroughs in the future. All in all, the quantum mechanical model of the atom is an incredibly important area of study that has the potential to transform the way we view the world and advance our understanding of the universe.